RIMS - Registration Information Management Software
Get an overview of registrations and all related information
A comprehensive registration information management solution designed for multinational companies and wherever large amounts of registration information need to be managed. The application is in English (globalized i.e. easy to prepare for any culture), accessible via the web. It is possible to purchase a permanent license or just rent it.
Basic workplace
The user is provided with a workplace that resembles Outlook. The basic folders are:
-
Active Ingredients,
-
National Registrations,
-
Products,
-
Registration Procedures.
The table with the found data allows to search using the filter line, new data are signalled in bold font. Red colour indicates a task for the current user, blue a group task and green a task due to a crowd. Double-clicking opens the corresponding record and the data can be updated.
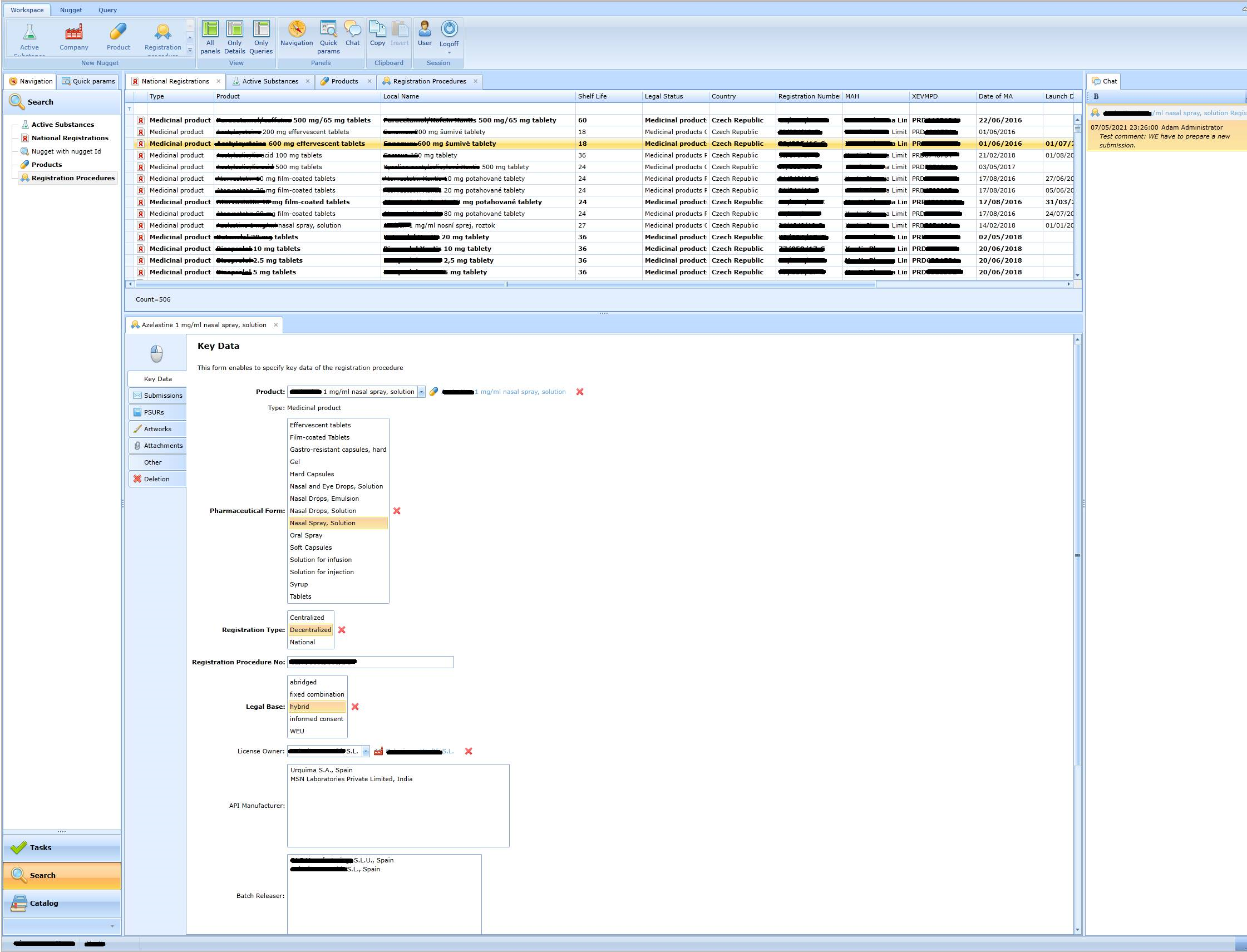
Records of active substances
The active ingredient is the key ingredient in the drug. It is used to further describe a medicinal product, which is a category of product. For example, it allows medicinal products to be grouped according to the active substances they contain.
Product records
The product is the product whose registration data we want to track. Can contain 0... n active ingredients. The key entry for a product is the type:
-
cosmetics,
-
food supplement,
-
medical device,
-
drug.
See other fields on the form:
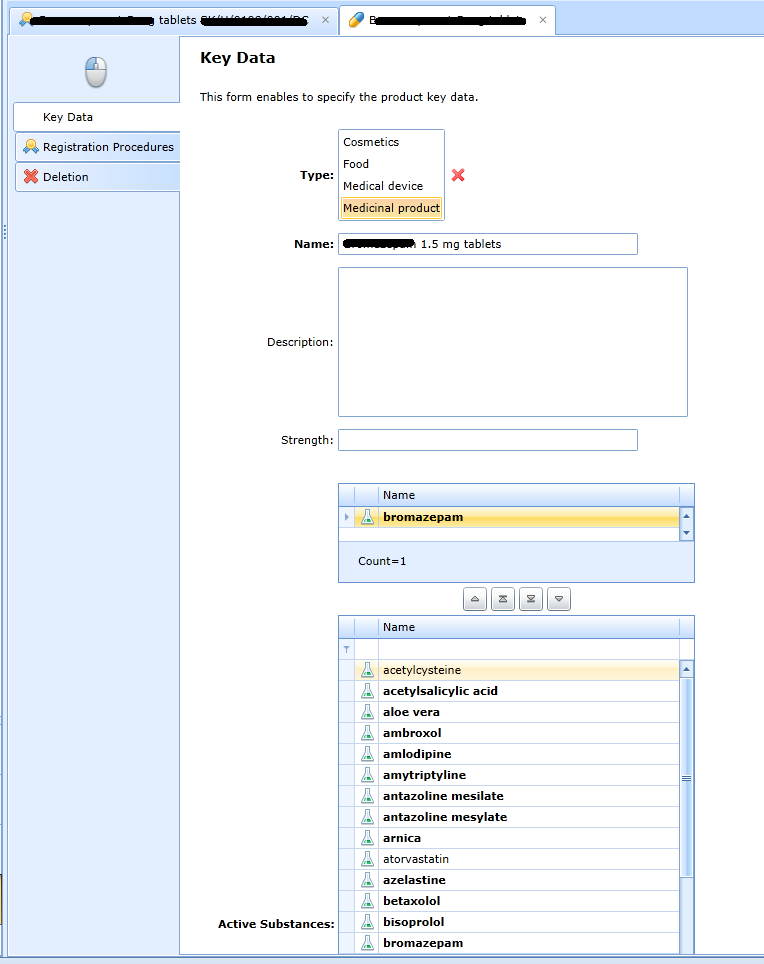
Records of registration procedures
The form varies according to the type of product (drug, cosmetic, medical device or food supplement). The form for a medicinal product looks like this:
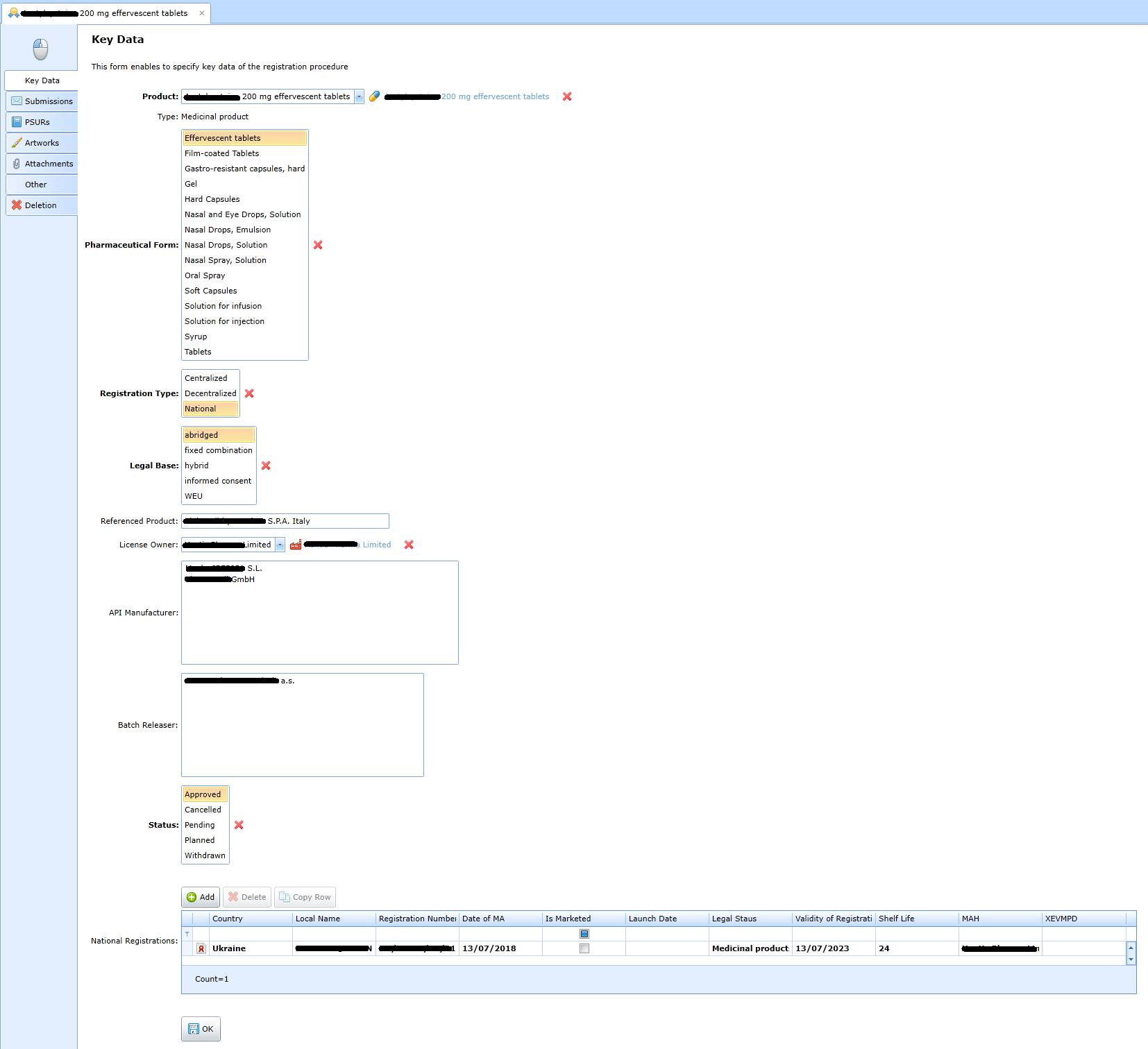
The registration procedure results in national registration in 0 .. n countries, see the following form:
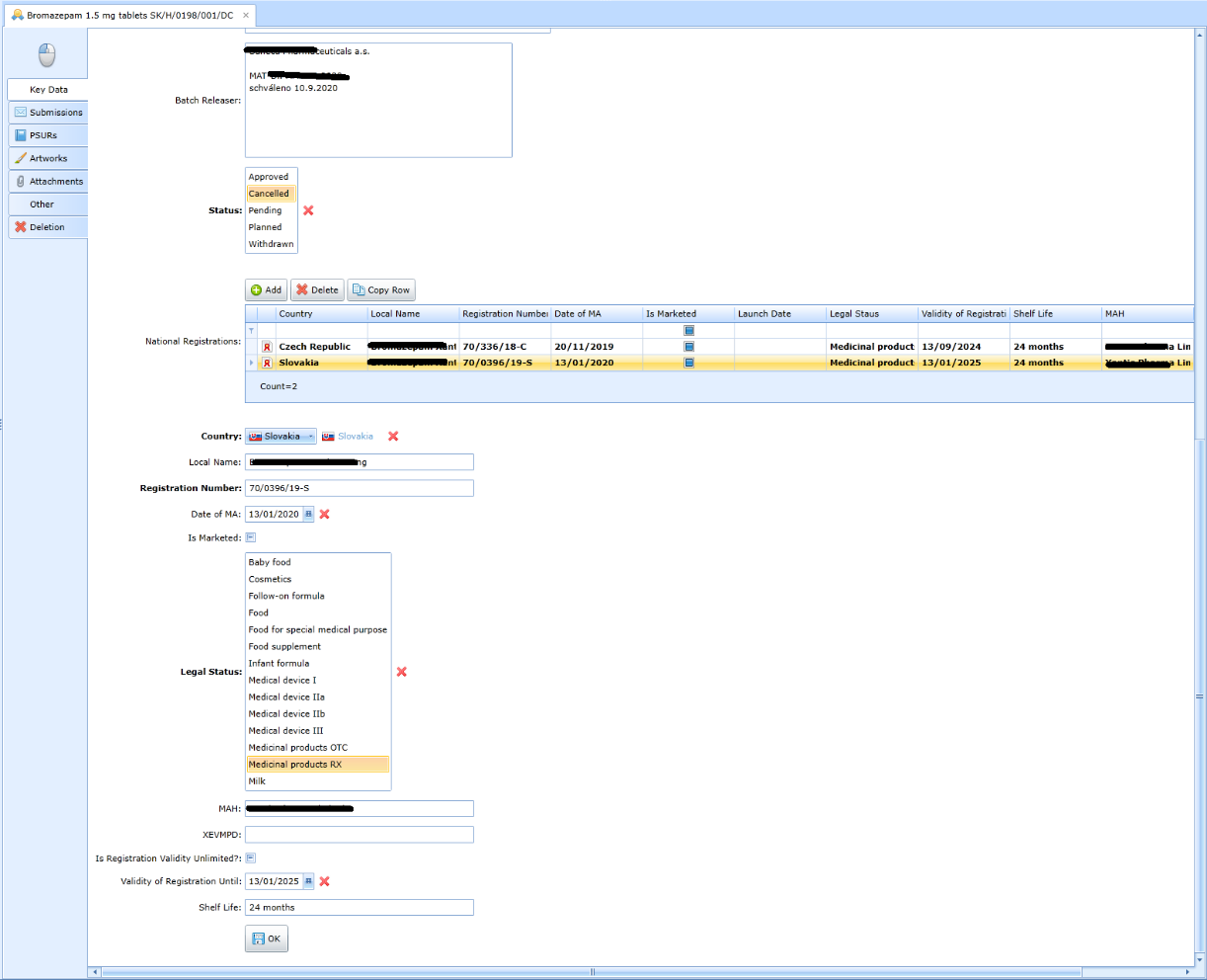
Registration of medicines is done by sending eCTD sequences. These are
are recorded on the Sumbissions form.
Records of submissions
ECTD sequences sent to the regulatory authority. In the EU, they may apply to multiple countries simultaneously. They can apply to multiple products at the same time.
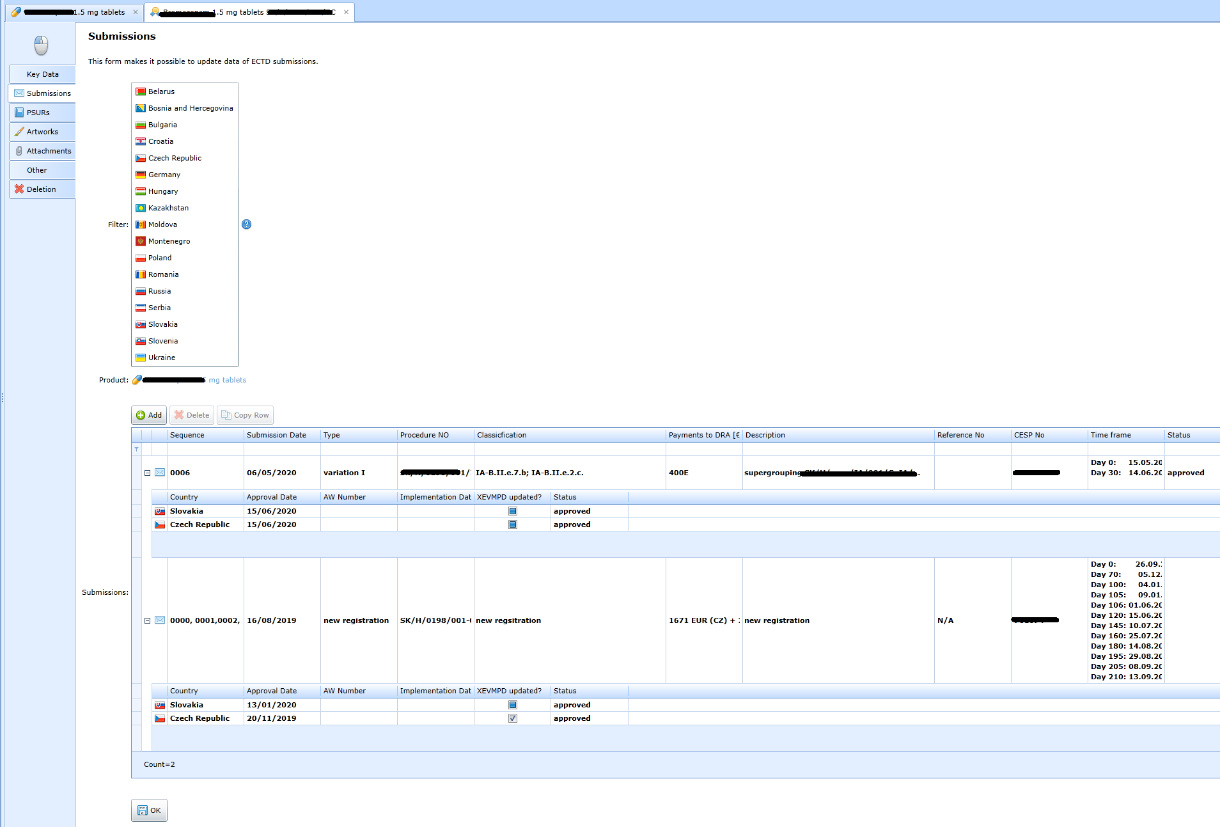
Details of the eCTD sequence.
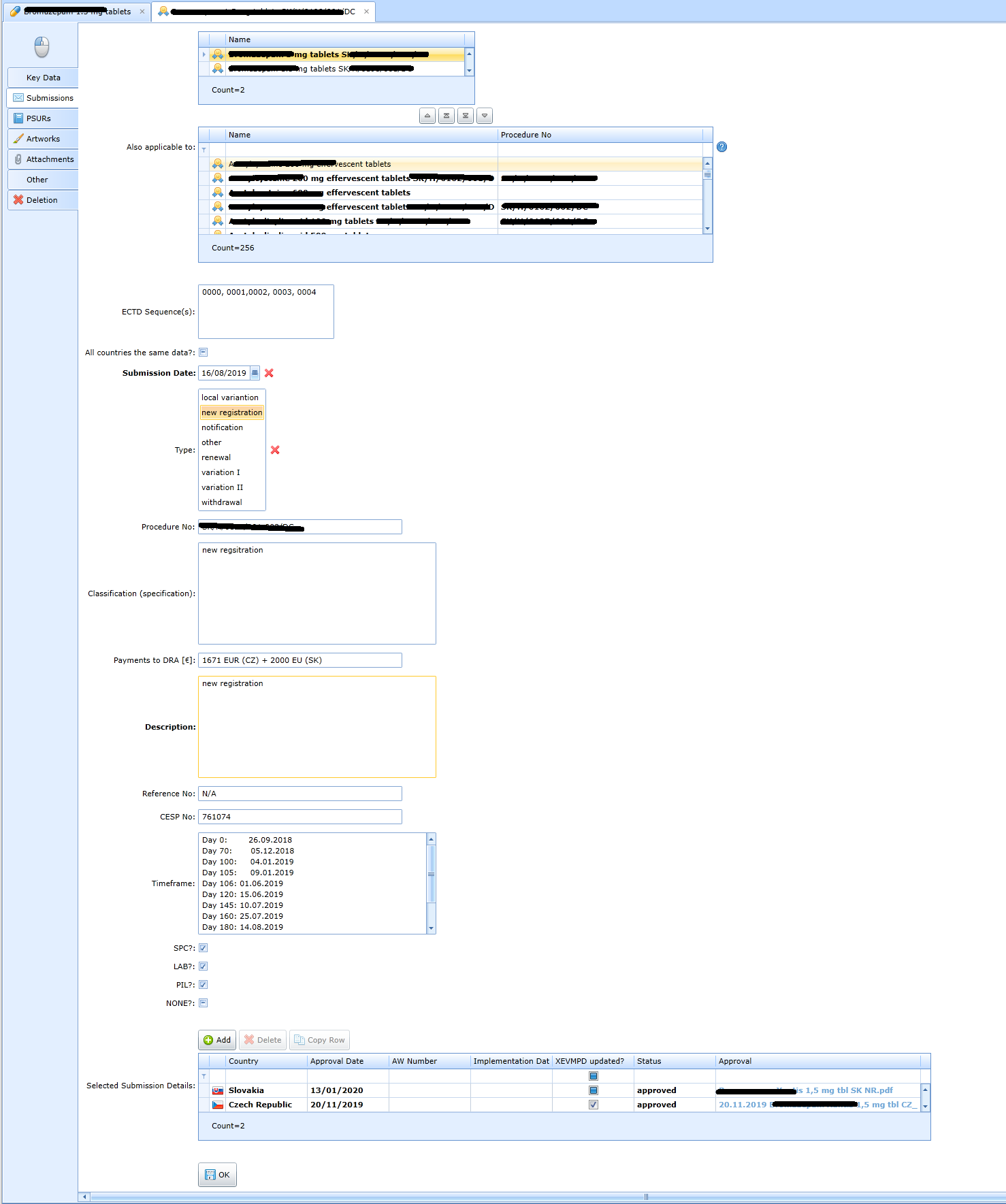
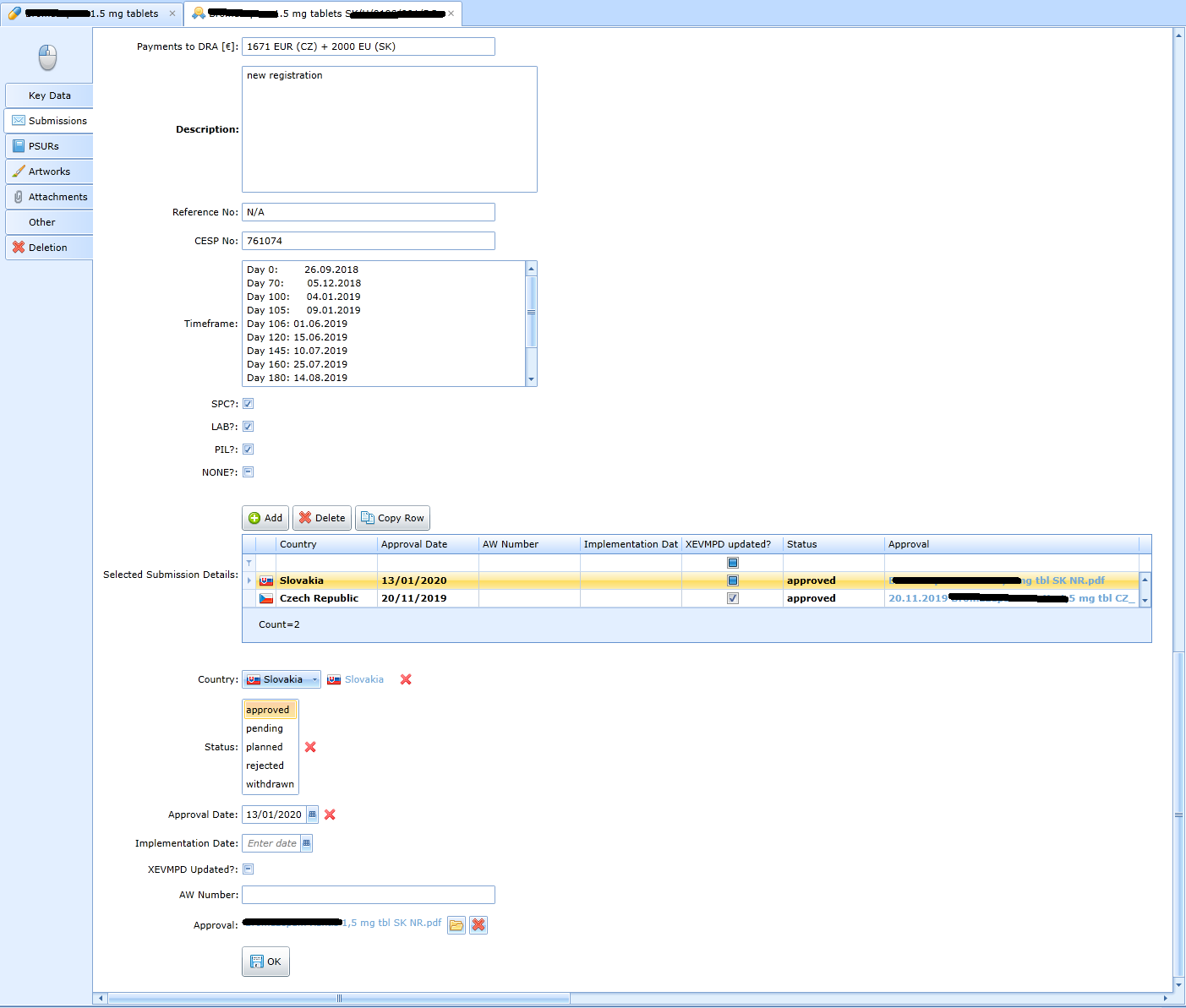
More detailed data on the status of processing in each country:
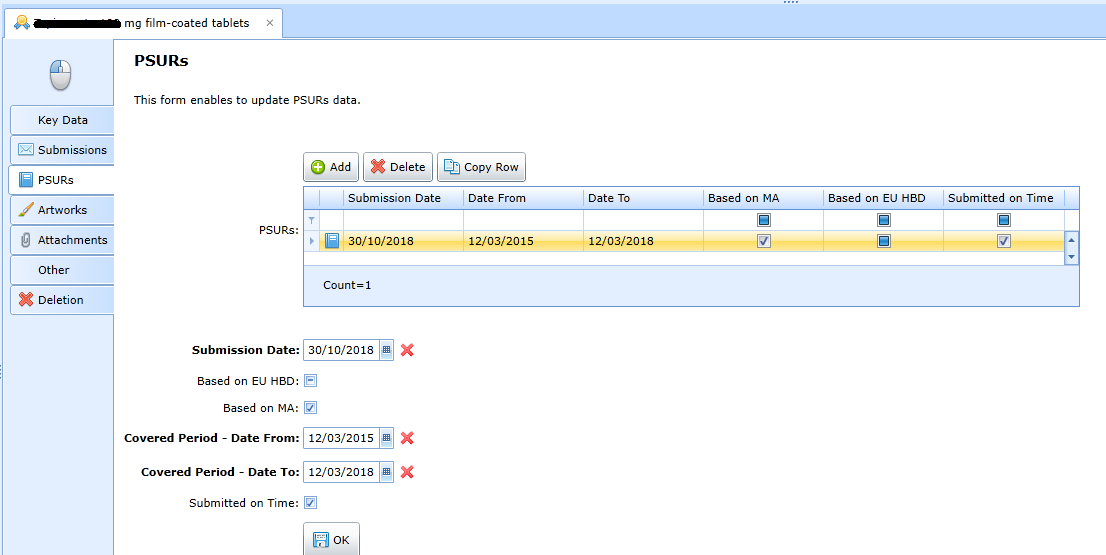
Records of PSURs
The application allows you to record data related to PSUR reports for each
registered product.
Artwork records (designs, 3D models of boxes, flyers)
The application allows you to keep files for each registered product of the following types:
-
blister, label,
-
box design,
-
PIL ...
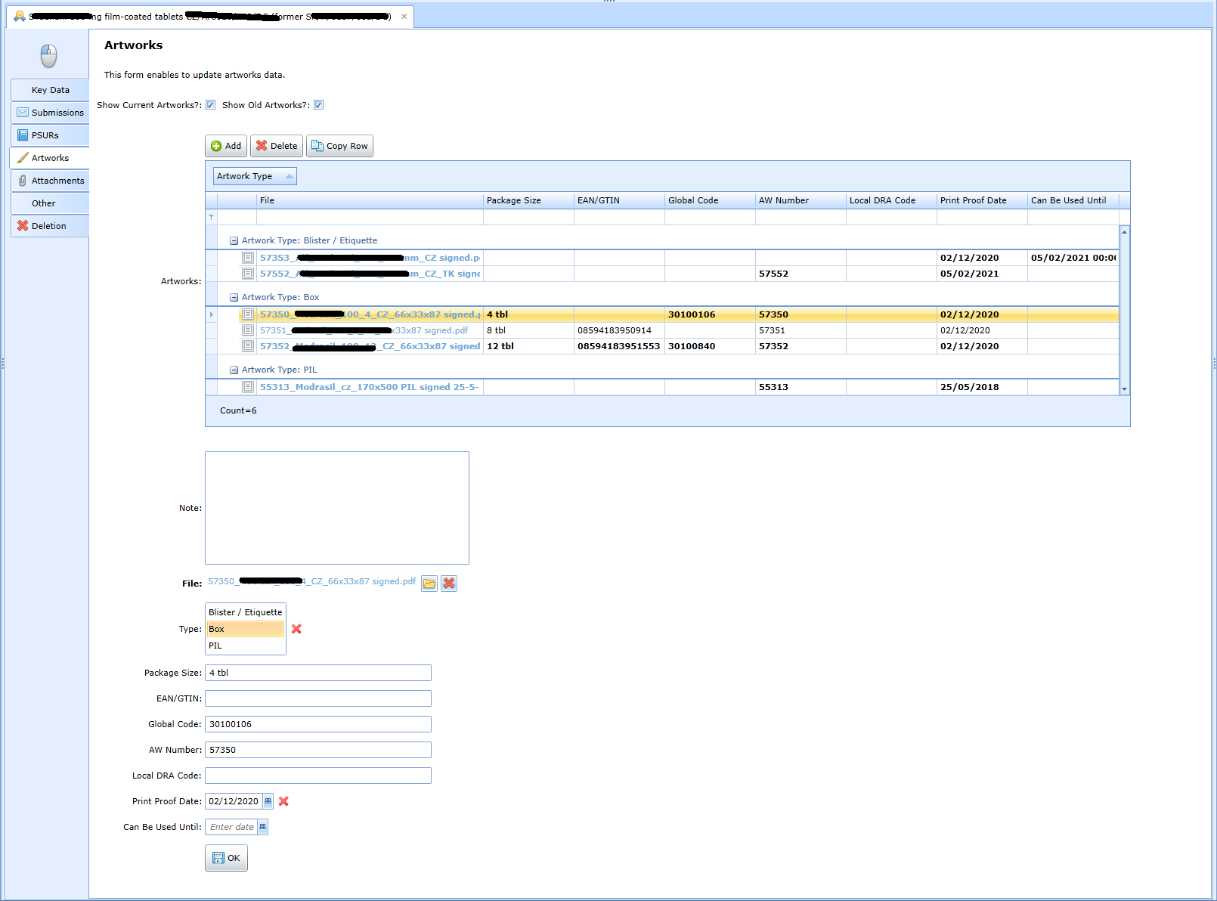
Evidence of attachments
Kromě předloh krabičky, příbalového letáku apod. je možné evidovat soubory dalších typů:
-
3d model,
-
local document,
-
a general common document,
-
MA.
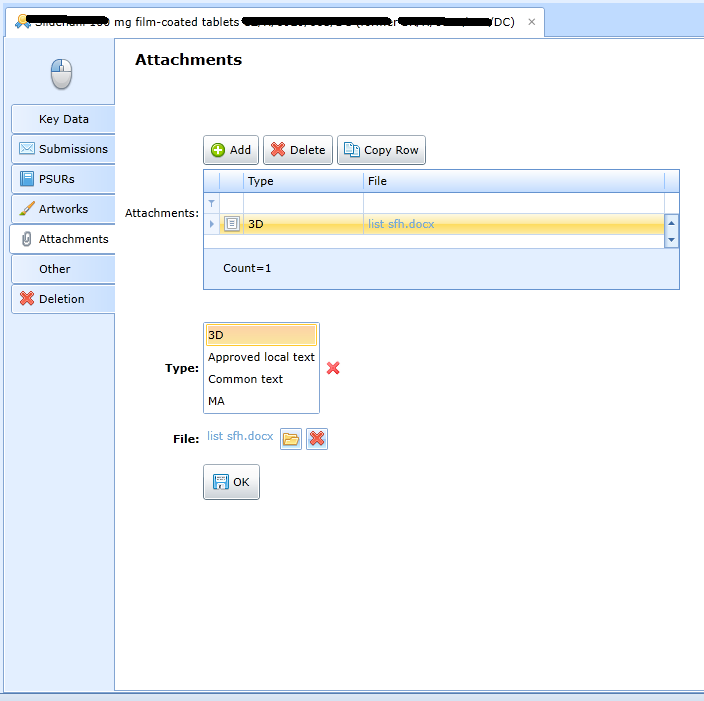
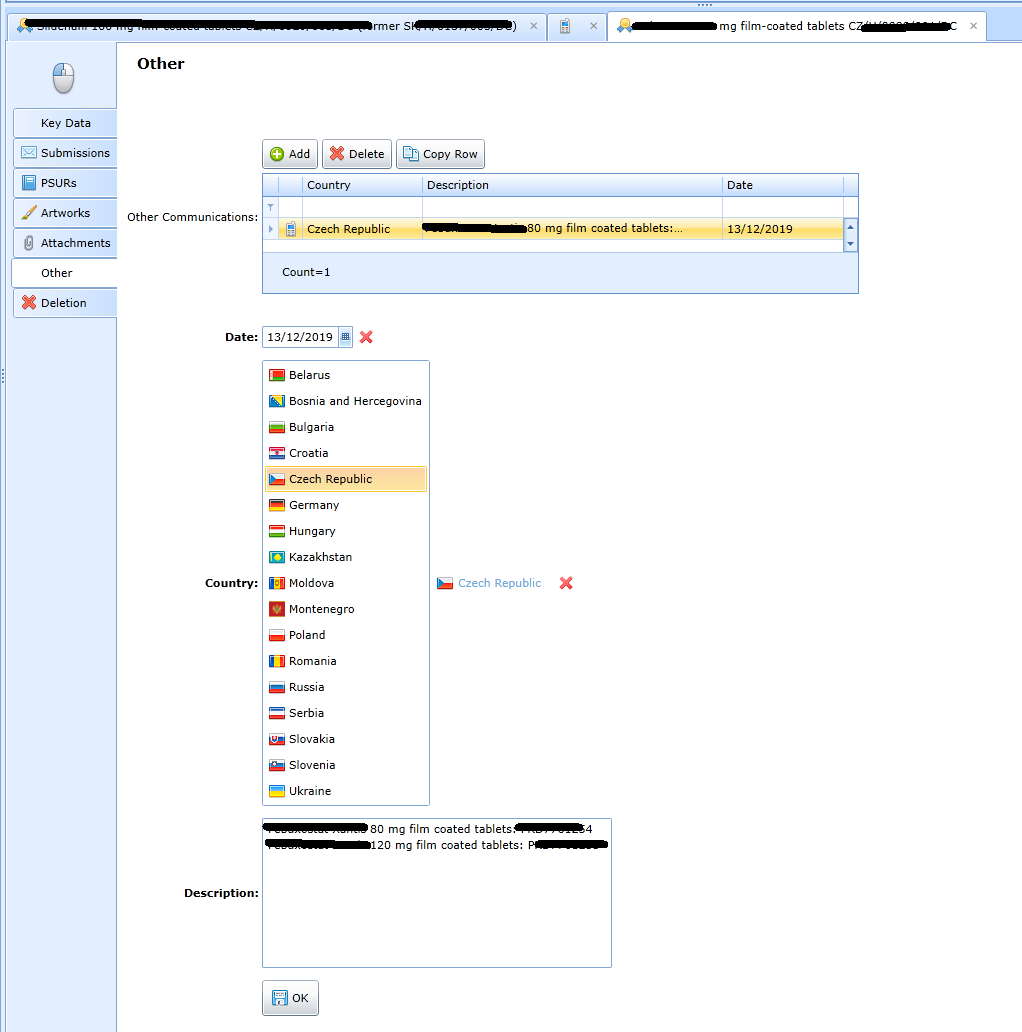
Evidence of remaining data and deadlines
The form is used to keep track of appointments and all other data that may be used by the user useful to the attendant.
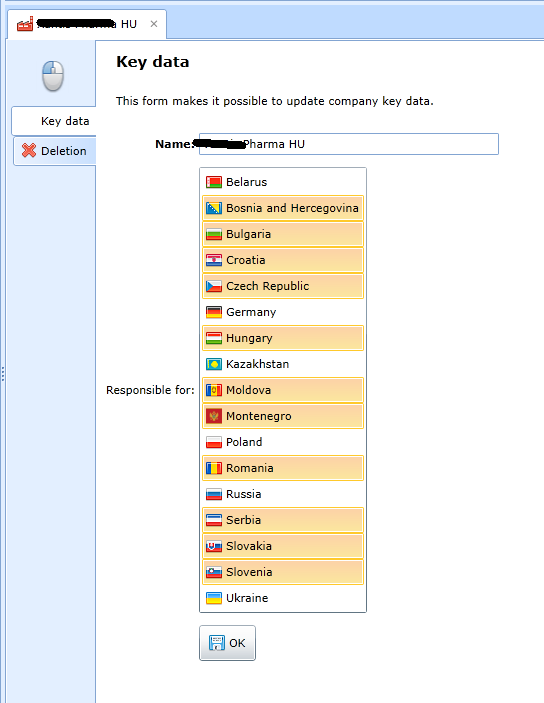
Security settings
The application allows you to define company branches and assign individual employees only according to the regional scope of the branch.
Readiness for change - Orgnes
The entire application is ready to go just by setting it up with the workflow engine Orgnes. Further changes can be made on the fly by simply changing the rules.
Technologie
Orgnes - RIMS module:
-
It is a Windows network application (accessible via the Internet).
-
It is installed without administrative permissions by simply clicking on a link in an email, for example.
-
The application server can be in the cloud or in your company.